Related Post
Reliable Cell Lysis Starts With Temperature Control: A Focused Ultrasonic Cell Disruptor for Sensitive Samples
2026-01-12Why Ultrasonic Heating Skews Results
When ultrasound is unfocused, heat becomes an unwanted co-reagent. Cavitation produces micro-scale hot spots that quickly raise temperatures, denature proteins, accelerate nucleases and proteases, and bias DNA fragmentation. This leads to drifting enzyme performance, shifted fragment size distributions, and batch-to-batch yield variability. Focused Ultrasonic Cell Disruptor technology confines cavitation and limits thermal stress to keep results consistent. Even when average bath temperature looks acceptable, thermal gradients inside the sample tube can be large enough to distort outcomes.
Even when the surrounding bath appears cool, transient temperature spikes inside the tube can:
•denature heat-sensitive proteins
•activate proteases and nucleases
•bias DNA or chromatin integrity
•reduce reproducibility between runs
These thermal artifacts propagate downstream. In next-generation sequencing (NGS), slight fragmentation bias can lead to increased GC content bias, higher repetition rates, and reduced complexity of the effective library. In proteomics, heat stress may favor abundant and thermostable proteins while reducing the abundance of low-abundance or thermosensitive targets. However, in the FFPE workflow, excessively high temperatures can exacerbate crosslink reversal variability and cause fragmentation levels to exceed the optimal range. The industry has long compensated with ice baths, intermittent pulsing, and trial-and-error timing – measures that reduce throughput but do not guarantee isothermal control at the sample core.
(Overcoming thermal weakening history in granite:
synergistic application of ultrasonic fatigue and residual heat – ScienceDirect)
What Is a Focused Ultrasonic Cell Disruptor
A Focused Ultrasonic Cell Disruptor concentrates high-frequency, short-wavelength acoustic energy into a focused acoustic zone that matches the sample location, rather than indiscriminately energizing the entire tank or a metal probe tip. Energy delivery is non-contact: acoustic waves couple through a controlled medium into a sealed sample vessel. This architecture avoids direct probe contact and minimizes aerosolization and cross-contamination risk while precisely governing how and where cavitation occurs.
At Longlight Technology, we engineered the acoustic path, the sample vessel interface, and the feedback control loop as one integrated system. A high-sensitivity temperature sensing and control module continuously monitors the actual sample zone – not just the bath – and adjusts power in real time to maintain true low-temperature, constant-temperature conditions. Because the focal geometry is stable, acoustic exposure becomes highly reproducible across runs and across instruments. The outcome is uniform disruption, predictable DNA or chromatin shearing, and consistent homogenization in an isothermal, non-contact environment that preserves molecular integrity.
- How Focused Energy and True Low–Temp Control Work?
•Confocal focusing: Short-wavelength ultrasound is precisely focused acoustically, concentrating energy in the sample while minimizing off-target losses.
•Isothermal environment: The process runs within a controlled water medium; heat extraction is immediate, preventing thermal spikes at the cavitation front.
•Closed-tube handling: Samples remain sealed; no probe insertion means lower contamination risk and no metallic microdebris.
•Real-time feedback: Temperature and power feedback stabilize process conditions, transforming a historically analog process into a digital, traceable one.
Evidence From the Field and International Studies
Focused ultrasonication has been widely adopted in molecular biology and sample preparation workflows where temperature stability and reproducibility are critical, including DNA shearing, chromatin preparation, and gentle cell lysis for proteomics.
• Meyer and Kircher (Nature Protocols, 2010) explain Illumina library construction that employs focused ultrasonication for precise DNA shearing, producing tight inserts for multiplexed sequencing and reducing thermal bias without sacrificing library complexity.
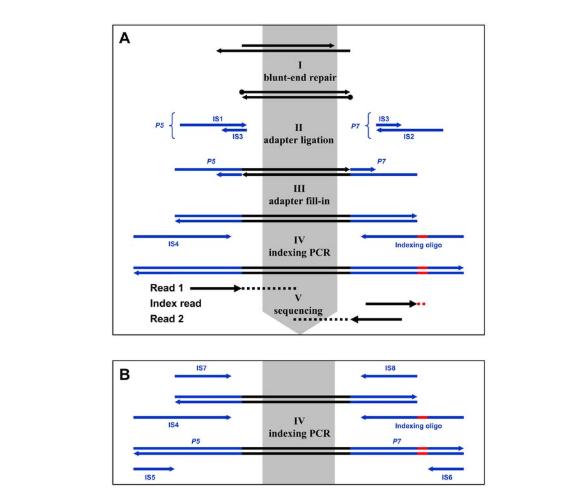
(Meyer and Kircher (Nature Protocols, 2010))
•Landt et al. (Genome Research, 2012), via ENCODE/modENCODE ChIP-seq guidance, emphasize controlled sonication (including focused modalities) to attain reproducible chromatin fragmentation, pressing for standardized energy input and careful temperature regulation to protect antibody-specific signals.
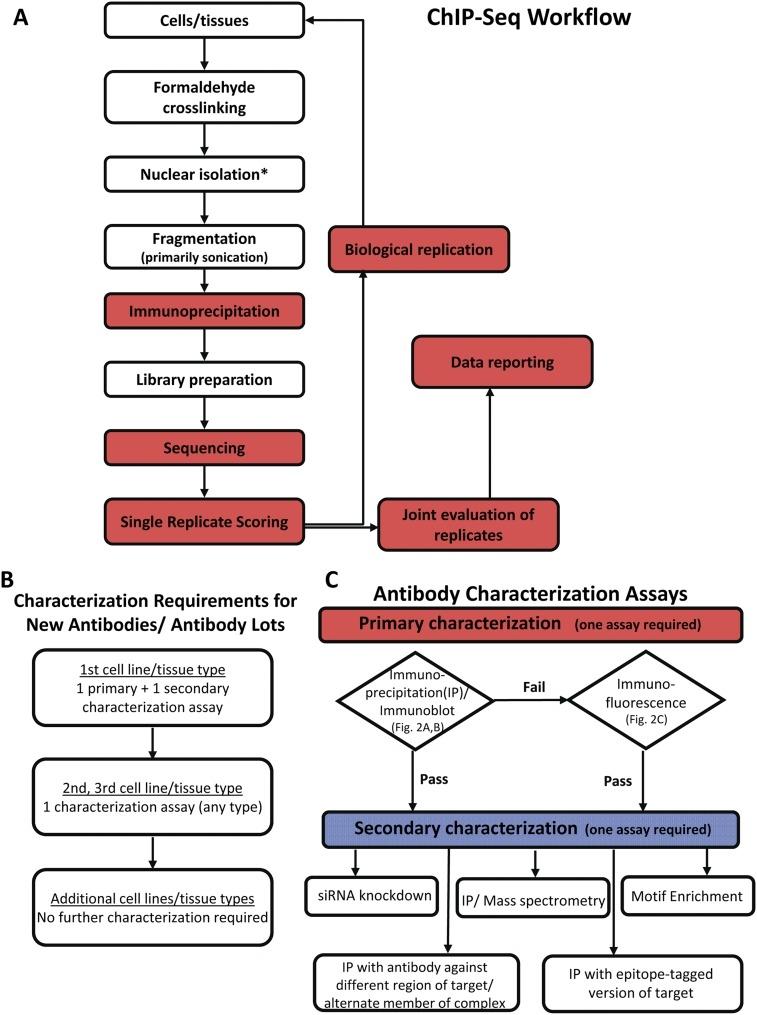
(Landt et al. (Genome Research, 2012))
•Van Dijk, Jaszczyszyn, and Thermes (Trends in Genetics, 2014) review NGS library construction and note that focused ultrasonication as a physical fragmentation approach produces narrower size distributions and curbs sequence bias relative to uncontrolled methods, particularly for GC-rich or degraded inputs.
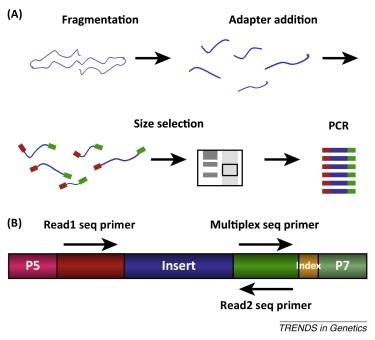
(Van Dijk, Jaszczyszyn, and Thermes (Trends in Genetics, 2014))
Across genomics, proteomics, and FFPE processing, the message is consistent: minimize thermal exposure and keep acoustic energy confined to the focal zone to improve reproducibility, refine fragment profiles, and enhance replicate agreement. These results validate the Focused Ultrasonic Cell Disruptor’s core principle: thermal discipline is the determinant of data integrity.
- NGS and Chromatin Shearing Benchmarks
•Tight fragment distributions reduce size-selection losses and boost library yield.
•Lower thermal drift preserves GC balance and lowers duplication rates.
•Reproducible chromatin fragments enhance peak calling and cross-site comparability.
Inside Longlight Technology‘s Solution
Longlight Technology built a desktop, multi–channel Focused Ultrasonic Cell Disruptor to make isothermal, non-contact acoustics accessible to every bench in the lab – without external sound enclosures or external computers. The platform supports eight sample positions and allows free mode processing from 1 to 8 samples, enabling independent conditions for each tube when your cohort is heterogeneous, and one-click batch mode when your run is uniform.
•Flexible throughput: Process 1 – 8 samples with independently customized acoustic exposure for complex cohorts, or input parameters once for a batch of similar samples to drive efficiency.
•Quiet by design: The engineered acoustic path and internal damping yield quiet operation without auxiliary sound covers, making it easy to deploy in communal labs.
•Non-contact processing: Energy is focused through a coupling medium into sealed vessels – no probe contact – lowering contamination risk and preserving sensitive materials.
•True low-temperature, constant-temperature control: High-resolution sensing plus closed-loop control maintain the setpoint in the sample zone, neutralizing process heat and protecting heat-labile analytes.
•Traceable records: Session metadata is logged and accessible at any time to enable auditability, SOP compliance, and data-driven optimization.
•Automated drainage: Single-tap waste discharge with smart level detection and early alerts averts overflow and keeps work surfaces clean.
•Built-in operating system: Operates without an external computer, shrinking the system footprint and simplifying validation.
•Built-in operating system: No external PC is needed, reducing footprint and streamlining IT validation.
At the acoustic core, our confocal focusing geometry concentrates power in the vessel interior, limiting energy loss and strengthening reproducibility. By removing the operator from manual timing and subjective “look and feel” decisions, the system elevates standardization across operators, shifts, and sites. It is particularly strong in DNA shearing for NGS, where uniform insert sizes translate directly into sequencing efficiency and cost control, and in chromatin shearing where antibody-specific signals can otherwise be masked by over- or under-fragmentation.

Common and Overlooked Knowledge that Solves Pain Points
•Temperature is local: Bath temperature is not sample temperature. Control must reference the sample zone to prevent hidden thermal gradients.
•Cavitation has a signature: Focused systems generate consistent bubble dynamics; erratic probe systems do not. Consistency translates into reproducible fragment distributions.
•Contamination is cumulative: Eliminating probe contact avoids gradual build-up of residues and metal particulates that can confound sensitive assays like MALDI-TOF MS.
•Logs matter: Traceable acoustic exposure and temperature profiles accelerate troubleshooting and enable defensible, regulatory-ready documentation.
From Pain Points to Payoffs: Applications and Outcomes
Many laboratories tolerate variability because legacy tools seem “good enough.” The cumulative cost – failed runs, repeats, and uncertain conclusions – outweighs the perceived savings. A Focused Ultrasonic Cell Disruptor reframes this calculus by combining acoustic precision with thermal discipline and operational simplicity.
Genomics and NGS: Controlled shearing produces narrow fragment distributions with minimal bias, improving cluster density, reducing adapter dimer formation, and enhancing effective library diversity. For GC-rich genomes or degraded DNA, true low-temp control preserves integrity during fragmentation.
Proteomics and Cell Biology: Non-contact disruption preserves native protein conformations and post-translational modifications that are susceptible to transient heating. The result is improved detection of low-abundance proteins and more faithful quantitative profiles.
Chromatin And Epigenomics: Reproducible chromatin fragment lengths strengthen peak resolution and reduce inter-run variability. Temperature stability protects protein – DNA complexes during lysis, preventing over-shearing and epitope loss.
MALDI-TOF MS Identification: For filamentous fungi and Mycobacterium, focused, non-contact energy accelerates cell wall disruption while minimizing contamination from probes, improving spectral clarity and pathogen identification confidence.
FFPE Deparaffinization And Extraction: Focused acoustics assist paraffin removal and crosslink management under tightly regulated temperatures, increasing nucleic acid and protein recovery without excessive fragmentation.
Operational Excellence: Eight-tube capacity with per-sample control or batch execution lets teams toggle between bespoke protocols and high-throughput pipelines. Quiet acoustics and a self-contained OS simplify siting in crowded spaces. Automated draining and searchable, traceable records trim manual work and support quality frameworks.
Call–to–Action
If your team is scaling NGS, proteomics, or clinical sample preparation, do not let ultrasonic heating dictate your data. Contact Longlight Technology to request a demonstration of our Focused Ultrasonic Cell Disruptor, review application notes for your assays, and evaluate true low-temperature control in your own workflows. Our specialists will map your current pain points to a validated acoustic method, enabling you to standardize results and accelerate time to insight.