Related Post
How XL-MS Accelerates Reliable Monoclonal Antibody Development
2025-11-18Monoclonal Antibody Development turns a single, well-defined antibody into a safe, scalable therapy. It spans discovery, engineering, analytics, and CMC. Teams apply it in oncology, autoimmune disease, and infectious threats. The field grew from the landmark work of Köhler and Milstein, and later advanced by global programs such as checkpoint inhibitors and COVID-19 neutralizing antibodies. Yet speed and certainty still clash. Structures shift. Interactions fade. Data arrives late or unclear. In this post, we follow a faster path. How can XL-MS expose the real binding story – without adding complexity – and set up the decisions that move a candidate forward?
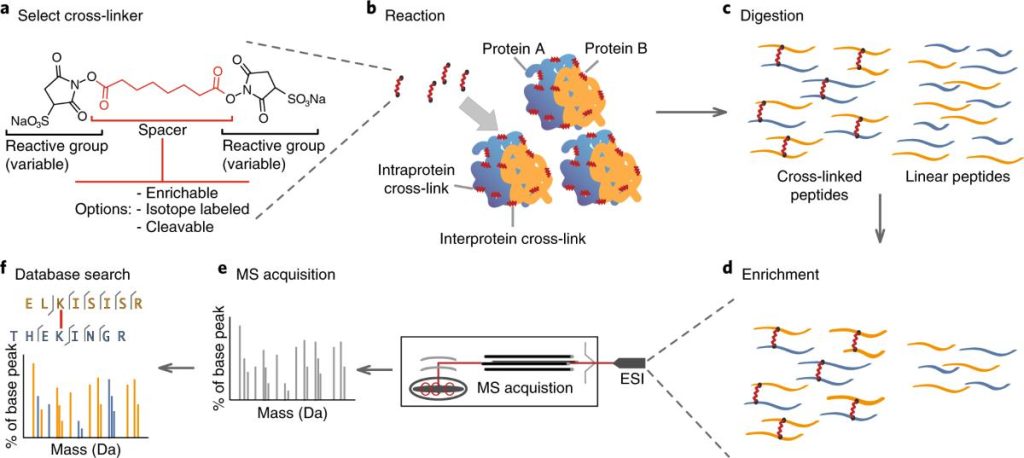
(Cross-linking mass spectrometry: methods and applications in structural, molecular and systems biology | Nature Structural & Molecular Biology)
The Real Bottleneck In Monoclonal Antibody Development
The central question is simple: how, exactly, does the antibody engage its antigen – and what else does it touch – in realistic conditions? Traditional structural tools remain essential, yet they are not always fast, tolerant of flexibility, or tuned to transient contacts. Material is often limited. Parallel assays compete for the same samples. Project teams need to triage candidates in days, not months.
XL-MS provides a practical path forward. Cross-linkers “lock” partners that sit within a defined proximity. Mass spectrometry then reads the cross-linked peptides and maps where proteins meet. These distance-aware constraints guide modeling, confirm epitopes and paratopes, and surface liabilities such as unexpected neighbors that could drive aggregation or off-target effects. In short, XL-MS gives Monoclonal Antibody Development programs the interaction clarity they need early enough to reshape the plan rather than explain the post-mortem.
- Why Traditional Tools Alone Aren’t Enough?
Crystallization can struggle with flexible domains. Cryo-EM can miss low-abundance or dynamic states. Computational predictions still require ground truth to avoid overfitting. XL-MS complements all three by preserving short-lived or weak interactions, working in complex mixtures, and delivering empirical constraints that feed directly into structural refinement. The outcome: faster convergence on the right lead and fewer surprises in developability.
How XL-MS Speeds Reliable Decisions
At Longlight Technology, our approach is to reduce friction and increase interpretability. XL-MS should not feel like a black box; it should read like a story you can defend in a project review. That is why we frame each run around a decision – validate an interface, confirm a mechanism, or de-risk a liability – then tune chemistry and analysis to answer that one question well.
✅ What XL-MS Reveals In Hours, Not Months
- High-throughput readouts that turn interaction maps quickly
- Compatible with intracellular or complex environments
- No special chemical labeling of proteins required
- Captures weak and short-lived contacts you would otherwise lose
These strengths align with the key decision points in Monoclonal Antibody Development:
- Epitope and paratope validation: Cross-links at or near the binding interface confirm intended recognition and reduce false positives from affinity-only screens.
- Mechanism of action clarity: Distinguish direct competition from allosteric effects when cofactors or multiple ligands are present.
- Liability screening: Reveal unexpected neighbors that may underpin aggregation, off-target binding, or Fc-mediated interactions before they surface in stress studies.
- Model refinement: Feed measured distance constraints into cryo-EM or X-ray pipelines to anchor flexible regions and accelerate final models.
XL-MS is not a silo. It strengthens the rest of the toolkit – structural, biophysical, and computational – so your narrative holds up across assays and across time.
From Sample To Insight: Our Service Workflow
Our delivery model is built for decisions, not just datasets.
- Plan: We define the hypothesis – interface mapping, complex stoichiometry, or developability risk – against your Monoclonal Antibody Development milestones.
- Prepare: Cross-linking under biology-relevant conditions, followed by enzyme digestion and peptide enrichment.
- Measure: High-resolution mass spectrometry with robust QC gates to ensure confident cross-link calls.
- Analyze: We map links, build interaction networks, and annotate plausible interfaces and constraints.
- Deliver: An interpretable report, figures suitable for slide decks, and recommended next experiments.
What does this mean in practice? A lead with verified epitope – paratope contacts moves faster into engineering. Compounds with risky neighbors exit the funnel early, saving material and meeting time. Structure teams gain restraints that reduce guesswork, while program managers gain a defensible timeline.
(Systems structural biology measurements by
in vivo cross-linking with mass spectrometry | Nature Protocols)
Beyond XL-MS: A Platform That Keeps You Moving
Monoclonal Antibody Development rarely lives in one lane. The same program that needs interface clarity also needs reliable genomics and lab infrastructure to validate targets, characterize cell lines, and keep routine steps tight and traceable. Longlight Technology supports that broader journey with solutions designed to lift both throughput and confidence.
✅ Genomics & Chromatin Context
- ChIP-seq enablement: Study protein – DNA binding across the genome, connecting antibody targets with chromatin context and regulation. This helps teams link a binding event to functional consequences in cells – useful when you must justify a mechanism to governance bodies.
- NGS-ready support: Our instruments and consumables for next-generation sequencing streamline library prep and run setup, so target validation and variant tracking do not become schedule risks.
✅ Reagents, Consumables, And Everyday Reliability
- Consumables and kits you can trust: Precast agarose gels, nucleic acid extraction kits, Qubit tubes, and library preparation kits built for consistency. When regulated workflows demand traceability, everyday reliability becomes a competitive advantage.
- Designed for pace and accuracy: Our laboratory reagents and instruments are selected to reduce repeats and minimize variability, so your team can spend more time interpreting results and less time rerunning them.
The thread through all of this is simple: pairing XL-MS interaction clarity with dependable genomics and lab infrastructure shortens the path to confident choices. That means fewer bottlenecks, cleaner gate reviews, and a smoother route to IND.
Call To Action: Turn Interface Clarity Into Program Velocity
If you’re ready to bring XL-MS into your Monoclonal Antibody Development workflow, partner with Longlight Technology. Request a planning session, a sample submission kit, or a pilot run aligned to your next milestone. We’ll help you replace structural blind spots with evidence you can act on – so the right candidate moves forward, and the rest of your pipeline moves with it.