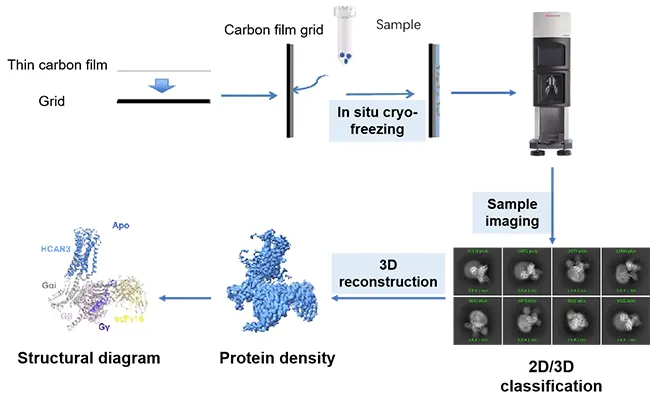
Related Post
Why XL-MS Accelerates Drug Target Discovery
2025-12-25The Drug Target Discovery is the process of finding the specific molecules in the body that a new medicine should act on. Successful cancer therapies, many kinase inhibitors, and immune checkpoint drugs like PD-1/PD-L1 antibodies all began with Drug Target Discovery breakthroughs. Researchers look for proteins that drive disease, validate their role, and then design small molecules or biologics to modulate them. Yet as diseases become more complex, traditional methods often struggle to reveal the right targets at the right time. How can we see protein interactions more clearly, in real biological environments, and move from candidate to confirmed target faster? This is where XL-MS begins to change the game…
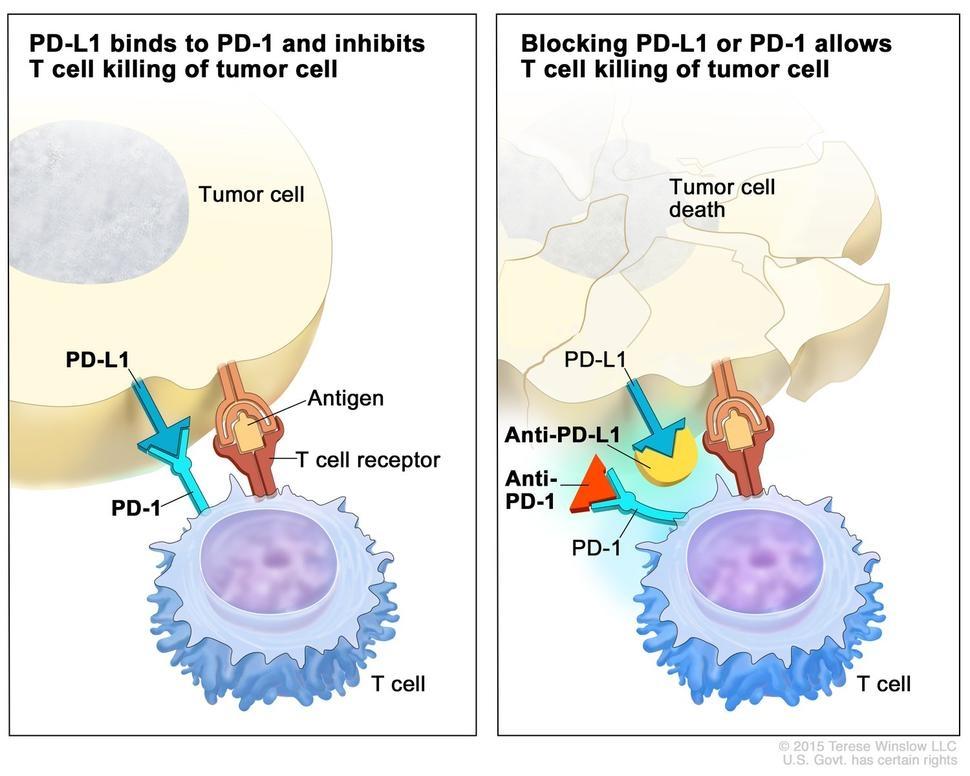
(Immune Checkpoint Inhibitors – NCI)
Seeing Drug Targets in Their Real Interaction Networks
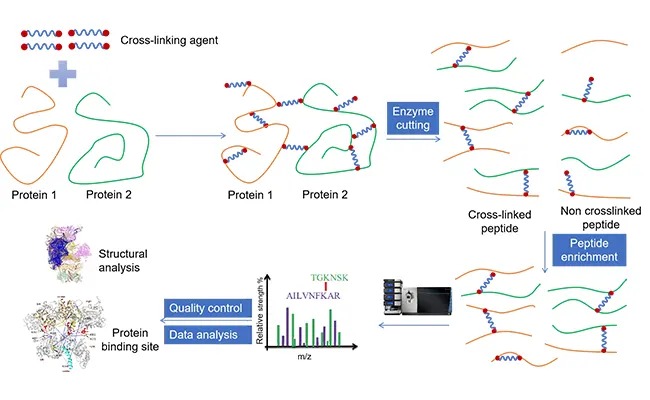
XL-MS (cross-linking mass spectrometry) starts from a very simple idea: if two proteins sit close enough to each other, a cross-linker can lock them together. Once that link is formed, mass spectrometry can detect the cross-linked peptides and show you which regions of the proteins are involved.
In practice, this means that you can add cross-linking agents directly to cell lysates or purified complexes. These reagents create covalent bonds between nearby amino acids, effectively “freezing” protein – protein interactions in place. Importantly, this includes weak, transient, or condition-dependent interactions that often disappear during purification or washing steps.
For Drug Target Discovery, that level of detail is extremely valuable. Most drug targets operate inside large multiprotein complexes or signaling pathways. If you only know the structure of a single protein, you see just one part of the story. With XL-MS, you can:
•Map which proteins directly interact with your target
•Locate approximate binding sites or domains involved in the interaction
•Compare interaction patterns between healthy and disease samples
•Track how candidate compounds might disrupt or stabilize specific contacts
XL-MS also combines well with structural methods like cryo-EM and X-ray crystallography. Structural biology gives you high-resolution snapshots. XL-MS adds distance restraints and interaction patterns that help validate or refine models, especially for flexible regions that are hard to resolve. Together, they provide a more realistic picture of how your drug target behaves inside a crowded molecular environment.
Why XL-MS Fits Modern Drug Target Discovery Pipelines
Many teams tell us the same thing: they want deeper insight into protein interactions, but they cannot afford a method that slows down the whole program. XL-MS is attractive because it delivers rich biological information without demanding a complete redesign of your workflow.
A few practical advantages stand out in day-to-day projects:
•High Throughput, High Information Content
XL-MS is well suited for complex samples and large studies. Once cross-linking and digestion are optimized, advanced mass spectrometers can handle many samples in a single run. Automated data processing then turns raw spectra into interaction maps, helping your team move faster from experiment to interpretation.
•Works In More Native Conditions
Because cross-linking can be performed in cellular or near-physiological environments, you are not restricted to highly purified proteins. This lets you capture interactions that only form in specific conditions, such as the presence of cofactors, membrane environments, or disease-relevant stress signals. For Drug Target Discovery, this means your candidates are evaluated in a context that better reflects what happens in vivo.
•No Need For Special Protein Labeling
XL-MS does not require custom chemical labels or genetic tags on each protein. That reduces experimental complexity, avoids potential artifacts from tagging, and makes it easier to scale across many targets and conditions.
•Sensitive To Weak And Transient Interactions
Some of the most important regulatory events involve low-affinity or short-lived complexes. These are exactly the types of interactions that can vanish with traditional pull-down or purification-based techniques. Cross-linking captures them at the moment they occur, giving you a more complete interaction network and helping you avoid blind spots during Drug Target Discovery.
Taken together, these benefits allow research teams to prioritize targets more intelligently. Instead of focusing only on what is easy to measure, you can focus on what is biologically meaningful – while still keeping timelines and budgets under control.
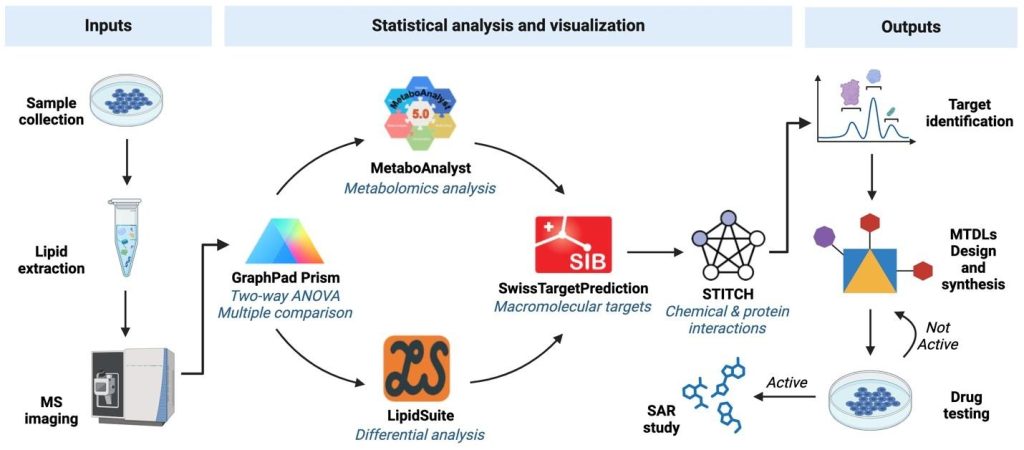
(Multi-Target Directed Ligands and Lipidomics Approach for Glioblastoma Drug Discovery)
How Longlight Technology Supports XL-MS Workflows End-To-End
At Longlight Technology, we position XL-MS not as a single experiment, but as part of an integrated solution for Drug Target Discovery. Many groups do not have the internal bandwidth to build a full XL-MS pipeline from scratch, so we design our services to plug into your existing research process.
You can either send us pre – cross-linked samples, or collaborate with our scientists to define a cross-linking strategy tailored to your system – choosing appropriate cross-linkers, conditions, and controls. From there, Longlight Technology takes care of every technical step, including sample digestion, cross-linked peptide enrichment, mass spectrometry acquisition, and data analysis. You receive a clear, project-ready report that maps protein interaction partners, identifies cross-link sites, and distills the structural information most relevant to your target and mechanism-of-action decisions.
Beyond XL-MS, Longlight Technology also supports the upstream and downstream work that surrounds Drug Target Discovery. Our genomics solutions, including next-generation sequencing – related instruments and reagents, help you identify genetic variants, expression changes, and regulatory elements linked to disease pathways. Techniques such as ChIP-seq connect protein – DNA interactions to the protein – protein interaction maps generated by XL-MS, giving you a broader view of how targets sit within chromatin regulation and transcription networks.
To keep your lab running smoothly, we also provide a range of consumables and kits – such as nucleic acid extraction kits, library preparation kits, precast agarose gels, and specialized tubes – that are designed for consistent, everyday use in research and biopharmaceutical environments. The mission is to simplify every stage – preparation, measurement, and analysis – so your scientists can spend less time on setup and troubleshooting, and more time testing hypotheses that matter. Bringing together XL-MS for interaction mapping, genomics platforms, and stable lab tools delivers a more coordinated and effective Drug Target Discovery workflow. Instead of isolated experiments, you build a pipeline that continually feeds better data into better decisions.
If your organization is looking to shorten discovery cycles, reduce risk, and gain a clearer picture of how potential drug targets behave in real biological systems, we would be glad to support you.
Interested in exploring XL-MS for your next Drug Target Discovery project?
Contact Longlight Technology to discuss your targets, samples, or study design, and let us help you turn complex protein interaction data into concrete, actionable insights.